Product Line-up for Medical Applications
AS of May 24 2024
*We plan to expand our selection of IEC60601-1certified power supplies for medical equipment.
| Series | Model | Medical Safety Agency IEC/EN60601-1 | Other Safety Agency Approvals | |
|---|---|---|---|---|
| MOPP | MOOP | |||
 GMA GMA |
GMA300F | ● | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 |
 GHA GHA |
GHA300F | ● | ● | C-UL, ANSI / AAMI ES60601-1, UL60950-1, EN62368-1 |
| GHA500F | ● | ● | ||
| GHA700F | ● | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 | |
 LMA LMA |
LMA100F | ● | ANSI / AAMI ES60601-1 | |
| LMA150F | ● | |||
| LMA240F | ● | |||
 AEA AEA |
AEA600F | ● | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1, EN62477-1 (OVCⅢ), UL508 (Option: -T5) |
| AEA800F | ● | ● | ||
| AEA1000F | ● | ● | ||
 PCA PCA |
PCA300F | ● | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 |
| PCA600F | ● | ● | ||
| PCA1000F | ● | ● | ||
| PCA1500F | ● | ● | ||
 PJMA PJMA |
PJMA300F | ● | ● | C-UL, ANSI / AAMI ES60601-1 |
| PJMA600F | ● | ● | ||
| PJMA1000F | ● | ● | ||
| PJMA1500F | ● | ● | ||
 AME AME |
AME400F | ● | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 |
| AME600F | ● | ● | ||
| AME800F | ● | ● | ||
| AME1200F | ● | ● | ||
 TUNS TUNS |
TUNS1200F | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 | |
 MHFS MHFS |
MHFS3 | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 | |
| MHFS6 | ● | |||
 MHFW MHFW |
MHFW3 | ● | C-UL, ANSI / AAMI ES60601-1, UL62368-1, EN62368-1 | |
| MHFW6 | ● | |||
MOOP(Means of Protection for Operators)
MOPP(Means of Protection for Patients)
Advantage of using Medical IEC60601-1 Approved Power Supplies
1. We contribute towards the reduction of the review period and expenses required in acquiring safety standards certification.
Safety standards are stipulated to guarantee the protection of people, property, and the environment. For medical devices it is required to meet the safety standards as stipulated by each country based on the IEC60601-1 medical electrical equipment standards as stipulated by the International Electrotechnical Commission (IEC). Compared to the commonly-known IEC6095-1 standards for information technology equipment, since medical devices involve the safety of people, the IEC60601-1 standards are much stricter.
In order to acquire safety certification it is necessary to test the equipment by utilizing a testing company. In the case medical equipment, of course it is necessary to test the therapeutic ability of the device as well as its safety, but the testing of safety for power supplies that primarily and secondarily insulate electrical potential is particularly strict and it is not unusual that testing takes several months since it is necessary meet the requirements for certification of internal components, submit test samples, confirm insulating distances/withstand voltage design, as well run the temperature rise and abnormality tests. If you choose to use our standard certified medical electrical equipment power supplies, it is generally not necessary to conduct safety standards testing and it is possible to reduce testing for primary and secondary electrical potential insulation. In addition, since our standard power supplies are constantly updated to meet the changing medical electrical equipment standards, we are also able to contribute towards reducing the amount of time needed for audits and adhering to updated regulations following mass production.
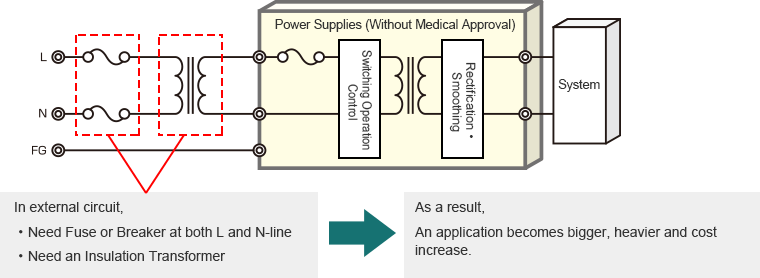
2. We contribute towards system miniaturization as well as weight and cost reduction.
In case of Power Supplies (Without Medical Approvals)
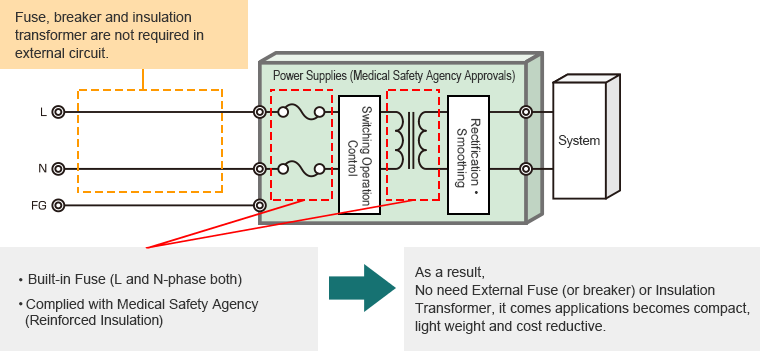
In case of Power Supplies (Medical Safety Agency Approvals)
Leakage Current Specifications
| Series | 100VAC input | 240VAC input |
|---|---|---|
| GHA (300 - 500W) | 0.125mA max | 0.250mA max |
| GHA (700W) | 0.10mA max | 0.20mA max |
| GMA | 0.13mA max | 0.30mA max |
| LMA | 0.10mA max | 0.25mA max |
| LMA (-G option) | 0.05mA max | 0.10mA max |
| PCA | 0.30mA max (reference) | 0.50mA max |
| PJMA | 0.15mA max (reference) | 0.30mA max |
| AME | 0.15mA max (reference) | 0.30mA max |
| AEA | 0.15mA max (reference) | 0.30mA max |










